

CIP #23- Discoloration
WHAT is Discoloration?
Surface discoloration is the non-uniformity of color or hue on the surface of a single concrete placement. It may take the form of dark blotches or mottled discoloration on flatwork surfaces, gross color changes in large areas of concrete caused by a change in the concrete mix, or light patches of discoloration caused by efflorescence. In this context, it is not intended to include stains caused by foreign material spilled on a concrete surface.
WHY does Discoloration Occur?
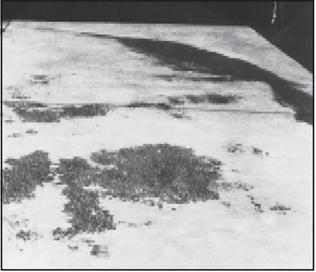
Discoloration due to changes in cement or fine aggregate sources in subsequent batches in a placement sequence could occur, but is generally rare and insignificant. Cement that has hydrated to a greater extent will generally be lighter in color. Inconsistent use of admixtures, insufficient mixing time, and improper timing of finishing operations can cause this effect. A yellowish to greenish hue may appear on concrete containing ground slag as a cementitious material. This will disappear with time. Concrete containing ground slag does, however, have a generally lighter color. The discoloration of concrete cast in forms or in slabs on grade is usually the result of a change in either the concrete composition or a concrete construction practice. In most studies, no single factor seemed to cause discoloration.
Factors found to influence discoloration are: the use of calcium chloride, variation in cement alkali content, delayed hydration of the cement paste, admixtures, hard-troweled surfaces, inadequate or inappropriate curing, changes in the concrete mix, and concreting practices / finishing procedures that cause surface variation of the water-cement ratio.
HOW to Prevent Discoloration?
► Minimizing or eliminating the use of high-alkali content cements will reduce the occurrence of discoloration.
► Calcium chloride in concrete is a primary cause of concrete discoloration. The chances for discoloration are much less if calcium chloride or chloride-bearing
chemical admixtures are not used.
► The type, kind, and condition of formwork can influence surface color. Forms with different rates of absorption will cause surfaces with different shades of color. A
change in the type or brand of a form release agent can also change concrete color.
► Eliminate trowel burning of the concrete. The most common consequence is that metal fragments from the trowel are embedded in the surface of the concrete.
Also, concrete which has been hard-troweled may have dark discoloration as a result of densifying the surface, which reduces the water-cement ratio. The
resulting low water-cement ratio affects the hydration of the cement ferrites, which contributes to a darker color. Concrete surfaces that are troweled too early will
increase the water-cement ratio at the surface and lighten the color.
► Concrete which is not properly or uniformly cured may develop discoloration. Uneven curing will affect the degree of hydration of the cement. Curing with
polyethylene may also cause discoloration. When the plastic sheeting is in direct contact with the concrete, it will cause streaks. Using an even application of a
quality spray or curing compound may be the better alternative.
► The discoloration of a slab may be minimized or prevented by moistening absorptive subgrades, following proper curing procedures, and adding proper protection of
the concrete from drying by the wind and sun.
HOW to Remove Discoloration?
Certain treatments have been found to be successful in removing or decreasing the surface discoloration of concrete flatwork. Discoloration caused by calcium chloride admixtures and some finishing and curing methods can be reduced by repeated washing with hot water and a scrub brush. The slab should be alternately flushed and brushed, and then dried overnight until the discoloration disappears.
If a discoloration persists, a dilute solution (1% concentration) of hydrochloric (muriatic) acid or dilute solutions (3% concentration) of weaker acids (such as acetic or phosphoric) may be tried. Prior to using acids, dampen the surface to prevent them from penetrating into the concrete, and flush with clean water within 15 minutes of application.
The use of a 20% to 30% water solution of diammonium citrate (2 lbs. in 1 gallon of water) has been found to be a very effective treatment by the PCA for more severe cases of discoloration. Apply the solution to a dried surface for 15 minutes. The whitish gel that forms should be diluted with water and brushed. Subsequently, the gel should be completely washed off with water. More than one treatment may be required.
Some types of discoloration, such as trowel burning, may not respond to any treatment. It may be necessary to paint or use another type of coating to eliminate the discoloration. Some types of discoloration may, however, fade with wear and age.
|
PRECAUTIONS
► Chemical methods to remove discoloration may significantly alter the color of concrete surfaces. Inappropriate or improper use of chemicals to remove discoloration may aggravate the situation. A trial treatment on an inconspicuous area is recommended. Acids should be thoroughly flushed from a concrete surface.
► The user of chemicals should refer to a Material Safety Data Sheet (MSDS) or manufacturer guidelines to be aware of the toxicity, flammability, and/or health hazards associated with use of the material. The appropriate safety procedures, such as the use of gloves, goggles, respirators, and waterproof clothing, are recommended.
|
References
1. “Surfaces Discoloration of Flatwork,” Greening, N. R. and Landgren, R., Portland Cement Association Research Department Bulletin RX 203, 1966.
2. “Discoloration of Concrete, Causes and Remedies,” Kosmatka, S. H., Concrete Products, April 1987.
3. “Discoloration of Concrete Flatwork,” Neal, R. E., Lehigh Portland Cement Company, 1977.
4. “Removing Stains and Cleaning Concrete Surfaces,” PCA, 1988.
5. “Discoloration: Myths, Causes and Cures,” Rech, D. P., Owl Rock Products, 1989.
6. “Removing Stains from Mortar and Concrete,” Corps of Engineers Miscellaneous Paper C-6808.
7. “Discolored Concrete Surfaces,” Goeb, Eugene O., Concrete Products, Vol. 96, No. 2, February, 1993.
1993
![]()